Catalog # CX1805
phospho-N-Cadherin (Tyr-820) Peptide
Blocking Peptide
| Blocking | 1:1000 |
| ELISA | 50 ng/well |
Size 50 μg
Cadherins are transmembrane glycoproteins vital in calcium-dependent cell-cell adhesion during tissue differentiation. Cadherins cluster to form foci of homophilic binding units. A key determinant to the strength of the cadherin-mediated adhesion may be by the juxtamembrane region in cadherins. This region induces clustering and also binds to the protein p120 catenin. The cytoplasmic region is highly conserved in sequence and has been shown experimentally to regulate the cell-cell binding function of the extracellular domain of E-cadherin, possibly through interaction with the cytoskeleton. Many cadherins are regulated by phosphorylation, including N-cadherin and E-cadherin. N-cadherin is phosphorylated by c-Src at Tyr-820, Tyr-853, Tyr-860, Tyr-884, and Tyr-886. Phosphorylation of Tyr-860 can disrupt cadherin binding to β-catenin. Since many of these tyrosine sites are conserved in the cadherin family, phosphorylation of these sites may be critical for cadherin function.
References
Qi, J. et al. (2006) Mol. Biol. Cell 17(3):1261.
Xu, Y. & Carpenter, G. (1999) J. Cell. Bioch. 75:264.
Xu, Y. et al. (1997) J. Biol. Chem. 272(21):13463.
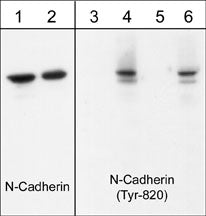
Western blot image of human endothelial cells untreated (lanes 1 & 3) or treated with pervanadate (1 mM) for 30 min (lanes 2, 4, 5 & 6). The blots were probed with anti-N-Cadherin (Cytoplasmic) (lanes 1 & 2) and anti-N-cadherin (Tyr-820) (lanes 3-6). The latter antibody was used in the presence of no peptide (lane 4), phospho-N-cadherin (Tyr-820) peptide (lane 5), or phospho-N-cadherin (Tyr-860) peptide (lane 6).
*For more information, see UniProt Accession P19022
The products are are safely shipped at ambient temperature for both domestic and international shipments. Each product is guaranteed to match the specifications as indicated on the corresponding technical data sheet. Please store at -20C upon arrival for long term storage.
*All molecular weights (MW) are confirmed by comparison to Bio-Rad Rainbow Markers and to western blot mobilities of known proteins with similar MW.
This kit contains:
